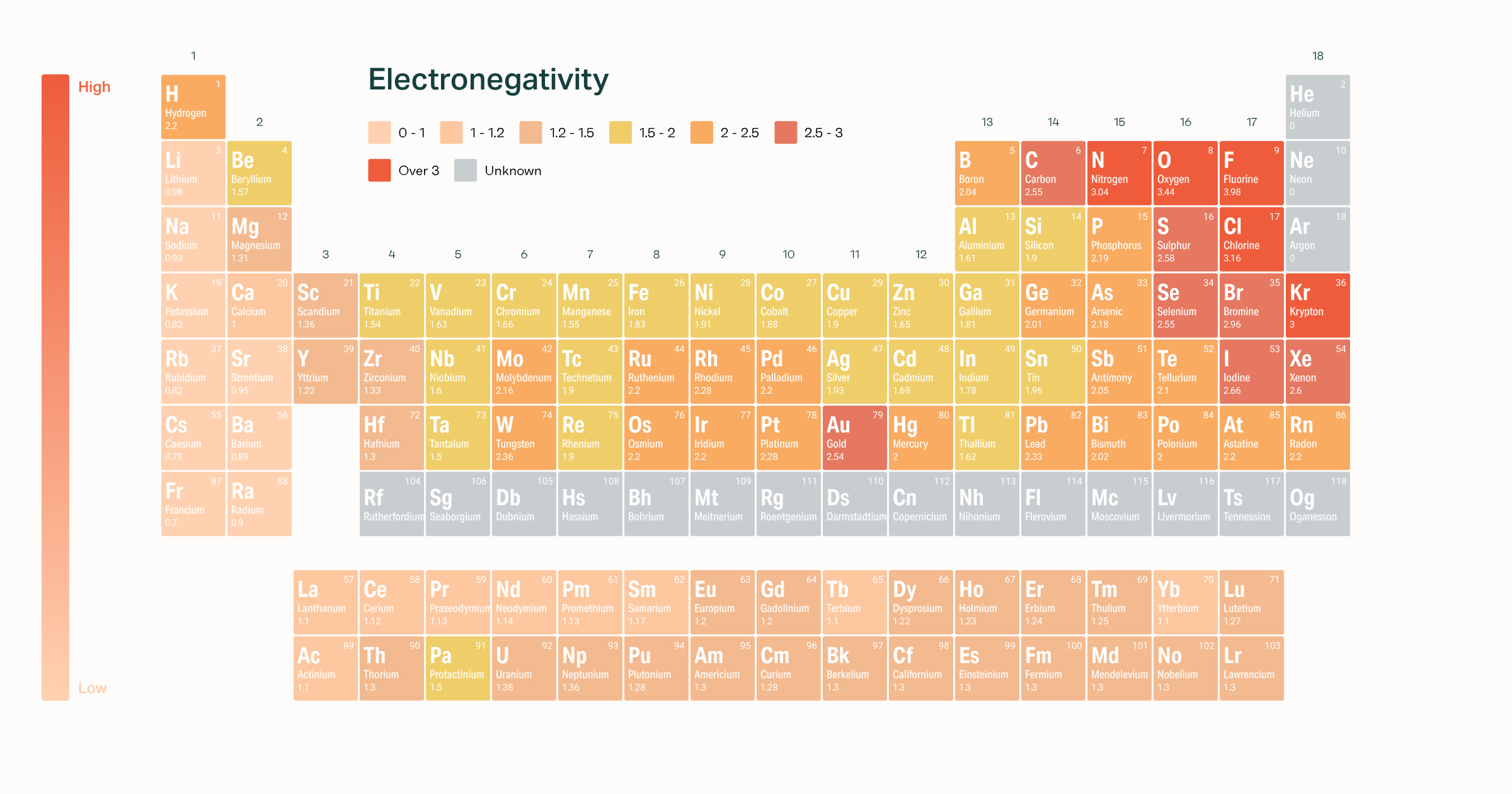
Based on these electronegativities GeH 4 would be expected to. The most electronegative element is Fluorine with a score of 40 the highest possible Across from Fluorine we also have N and O with high electronegativities.
Of the following elements which has the highest electronegativity.
. Dont hesitate to ask questions and start. Which of the following elements has the highest electronegativity. Arrange aluminum boron nitrogen and phosphorous in order of increasing electronegativity.
- electronegativity generally increases from left to right and from bottom to top of the periodic table. Helium neon and argon are not listed in the Pauling electronegativity scale although in the Allred-Rochow scale helium has the highest electronegativity. So since elements like the alkaline earths that form cations have low electron affinity it should make sense to you elements that form anions tend toward high electron affinity.
A simple way to think about. The amount of energy released when an atom gains an electron per mole. Br C Nb Sb or Cs.
Lowest electronegative element is Francium or Cesium as it is located at the bottom of the first period. Fluorine is the most electronegative element. Flourine has the highest and Francium the lowest electronegativity.
119 rows ELEMENT ELECTRONEGATIVITY. Of the following elements which has the highest electronegativity. The ability of an atom in a bond to draw electrons to itself.
Of the following elements which has the lowest electronegativity. Which of the following elements would be expected to have the highest electronegativity. Electronegativity refers to the ability of an atom to attract shared electrons in a covalent bond.
Hence option D is correct. A Electronegativity decreases down the group therefore boron has the highest electronegativity. Advertisement Answer 50 5.
Group 18 elements have no electronegativity. Electronegativity Which of the oxidation states of chromium has the largest valence-state electronegativity. Elements from the halogen group including F Cl Br have pretty high electronegativities.
Please explain and thanks. The amount of energy required to remove an electron from an atom per mole. Fluorine has the highest electronegativity 40and caesium the lowest 079.
Electronegativity is basically how much elements want electrons. Electronegativity increases from bottom to top in groups and increases from left to right across periods. Thus fluorine is the most electronegative element while francium is one of the least electronegative.
As we move right in a period the electronegativity keeps increasing up to the halogens. The noble gases ie. Which of the following elements has the highest electronegativity.
Which of the Following Elements Has the Highest Electronegativity. Ge Al P Li As P. This is because the number of electrons keeps increasing in the same valence shell so hold of the nucleus on the electrons is tight.
The elements are present in 3 r d period in the periodic tableOn moving across the period from left to right the electronegativity increases because the elements on the right side of the periodic table have more tendency to attract the electron in a bond so as to complete their valence shell of 8 electronsThus S is the most electronegative element. Of the following elements which has the highest electronegativity. Of the following elements which has the highest electronegativity.
Based on these electronegativities SiH4 would be expected to have polar covalent bonds with a partial negative charges on the H atoms. The higher the value of the electronegativity the more strongly that element attracts the shared electrons. What 3 elements have the highest electronegativity.
The highest electronegative element is Flourine as it is located in the right side of the periodic table. S Sc As P S Of the following elements which has the lowest electronegativityMg Cl Br Ca Ca The electronegativity is 21 for H and 18 for Si. The correct answer is option 1 ie.
Period in the periodic tableOn moving across the period from left to right the electronegativity increases because the elements on the right side of the periodic table have more tendency to attract the electron in a bond so as to complete their valence shell of 8 electronsThus S is the most electronegative element. Fluorine has the highest electronegativity followed by chlorine bromine and then with the least reactivity we have iodineamongst the given options. The potential energy that results from the interaction of charged particles.
The charge of one electron. The electronegativity is 21 for H and 18 for Ge. Our experts in all academic subjects are available 247.

The Parts Of The Periodic Table

Electronegativity Of The Elements

What Is The Periodic Trend Of Electronegativity Quora
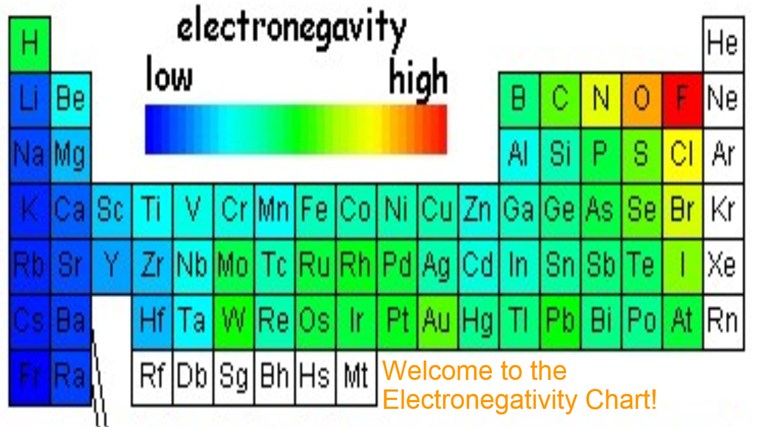
Electronegativity Chart Of Elements List Of Electronegativity
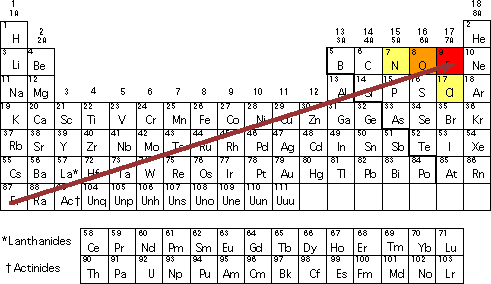
0 Comments